Optimization of Allosteric With-No-Lysine (WNK) Kinase Inhibitors and Efficacy in Rodent Hypertension Models.
Yamada, K., Levell, J., Yoon, T., Kohls, D., Yowe, D., Rigel, D.F., Imase, H., Yuan, J., Yasoshima, K., DiPetrillo, K., Monovich, L., Xu, L., Zhu, M., Kato, M., Jain, M., Idamakanti, N., Taslimi, P., Kawanami, T., Argikar, U.A., Kunjathoor, V., Xie, X., Yagi, Y.I., Iwaki, Y., Robinson, Z., Park, H.M.(2017) J Med Chem 60: 7099-7107
- PubMed: 28771350
- DOI: https://doi.org/10.1021/acs.jmedchem.7b00708
- Primary Citation of Related Structures:
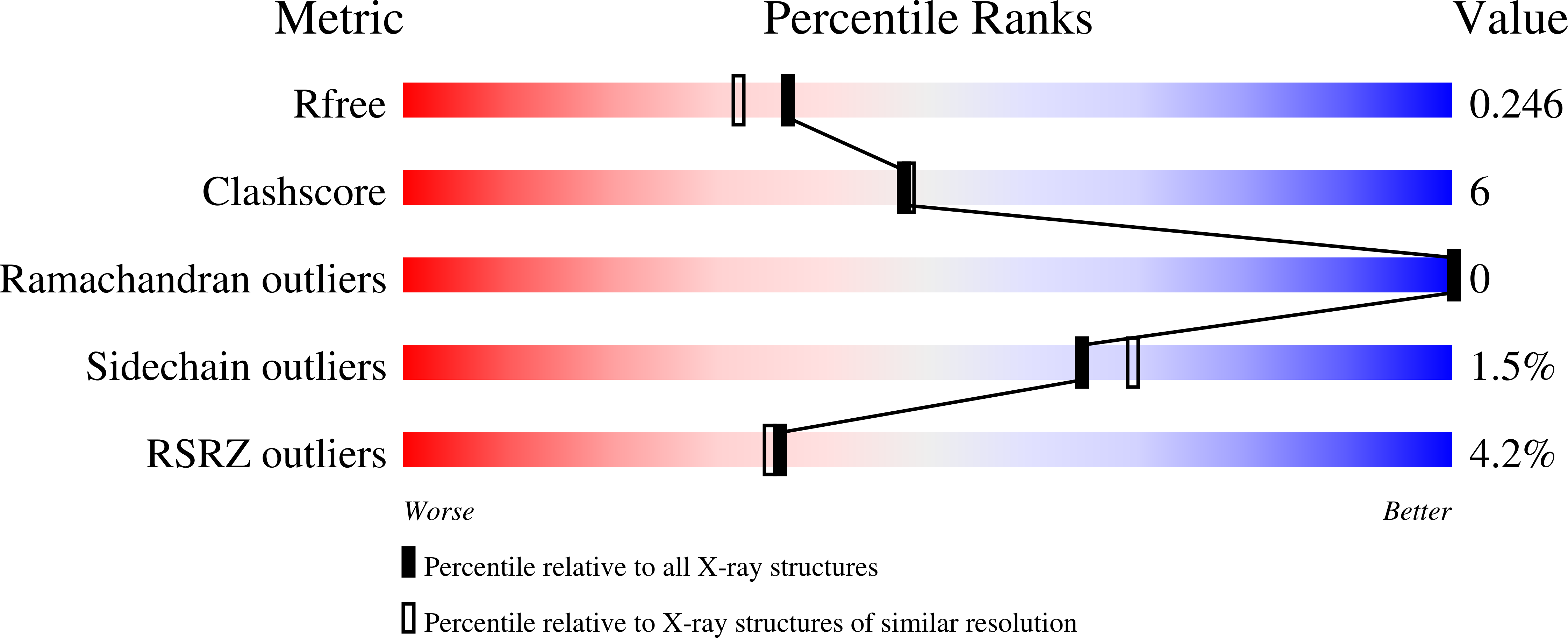
5WDY, 5WE8 - PubMed Abstract:
The observed structure-activity relationship of three distinct ATP noncompetitive With-No-Lysine (WNK) kinase inhibitor series, together with a crystal structure of a previously disclosed allosteric inhibitor bound to WNK1, led to an overlay hypothesis defining core and side-chain relationships across the different series. This in turn enabled an efficient optimization through scaffold morphing, resulting in compounds with a good balance of selectivity, cellular potency, and pharmacokinetic profile, which were suitable for in vivo proof-of-concept studies. When dosed orally, the optimized compound reduced blood pressure in mice overexpressing human WNK1, and induced diuresis, natriuresis and kaliuresis in spontaneously hypertensive rats (SHR), confirming that this mechanism of inhibition of WNK kinase activity is effective at regulating cardiovascular homeostasis.
Organizational Affiliation:
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States.